FERMO Brew Acid is an active dry yeast strain that produces lactic acid during fermentation. Sour beers are a vibrant and evolving category, capturing the imagination of brewers and drinkers alike. But traditional methods of souring; kettle souring, spontaneous fermentation (click here to read more about this) often introduce risks and inconsistencies. FERMO Brew Acid provides an optimised way to brew sours with a clean, direct, and controllable method for acidification through primary fermentation.
What FERMO Brew Acid Can Do: Performance & Fermentation Profile
FERMO Brew Acid - a unique yeast for a clean controlled sour beer fermentation
Lactic Acid Production
FERMO Brew Acid generates lactic acid during fermentation, contributing to a rounded, tangy acidity. This acidification happens in tandem with alcohol production, eliminating the need for bacteria like Lactobacillus.
Clean, Balanced Fermentation
Despite its acidity, this strain produces minimal hydrogen sulfide (H₂S) and is non-phenolic (POF⁻), meaning it won’t introduce medicinal or clove-like off-flavours. The ester production is moderate, resulting in subtle fruity/floral undertones, letting adjuncts (like fruit or spice additions) shine through.
High Flocculation & Medium Attenuation
With high flocculation, this yeast settles well post-fermentation, aiding in beer clarity. Attenuation levels of 75–80% make it suitable for a range of sour beer styles while retaining some body and sweetness unless blended with high attenuating strains for drier profiles.
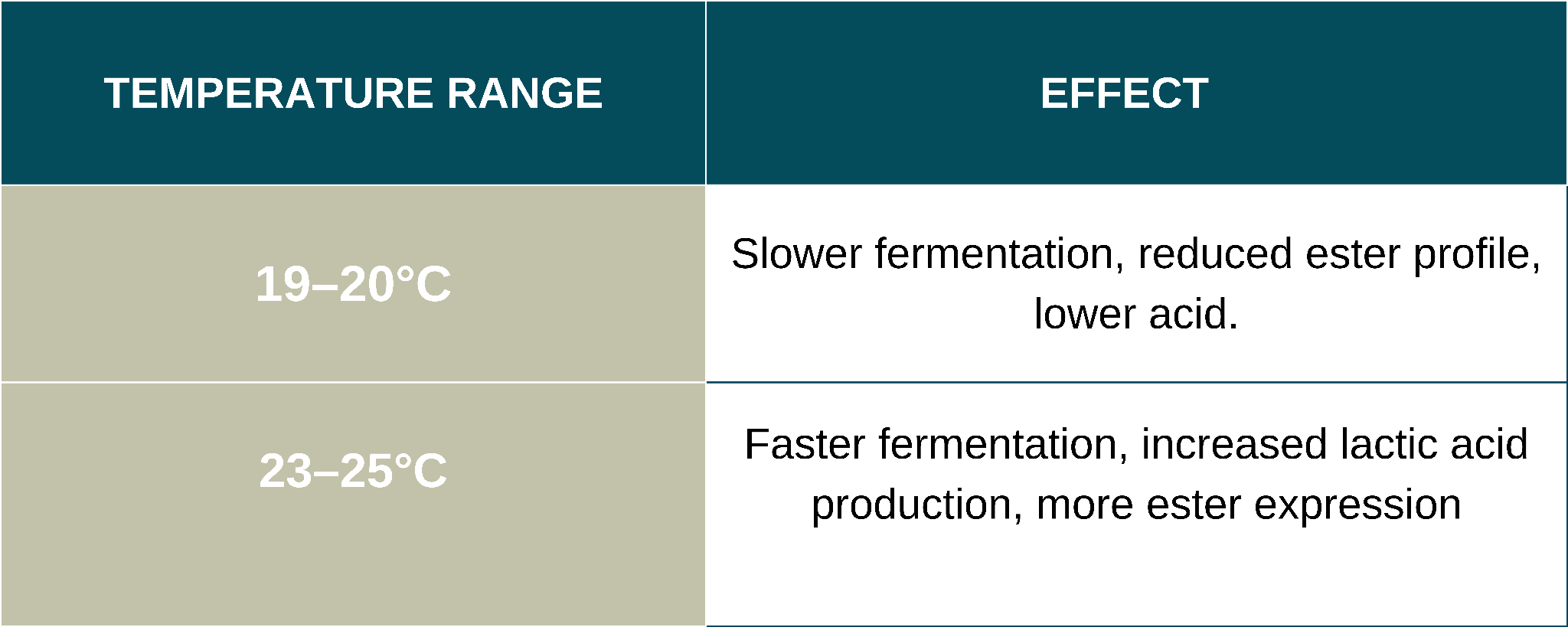
Effect of Fermentation Temperature (19–25°C)
FERMO Brew Acid thrives within a temperature range of 19–25°C, the table below summarises the effect when fermenting on the lower and higher end of the range.
For higher acidity and fruity complexity, ferment closer to 24–25°C. For a more restrained profile, target 19–20°C.
Maintaining consistent temperatures within a batch is crucial. Fluctuations can stress the yeast, impacting viability, acid production, and fermentation kinetics.
How to Increase Final pH (Reduce Acidity)
If you wish to reduce the final acidity or raise the pH of a brew using FERMO Brew Acid:
Shorten fermentation time before chilling: Pulling the beer early can limit lactic acid buildup.
Adjust mash pH or water chemistry: Start with a higher wort pH (e.g., 5.4–5.5 instead of 5.2) to buffer acidity.
Monitoring and adjusting the buffering capacity of the wort can help tailor the pH drop to your target.
Fermenting to Full Attenuation
FERMO Brew Acid is moderate in attenuation (75–80%), which may leave residual sugars depending on the wort composition. To achieve full attenuation (especially for drier or mixed-ferment beers):
Apply nutrient management (especially in adjunct-heavy or high-gravity worts). Click here to learn more about our yeast nutrition solutions
Ensure complete wort oxygenation at pitch
Consider increasing the pitching rate to 100 g/hL in more demanding fermentations (high gravity, low pH, or high adjunct load)
References
AEB Group. (2023). FERMO Brew Acid Technical Data Sheet (Australia). Retrieved from internal document provided by user.
Canonico, L., Comitini, F., Ciani, M., & Oro, L. (2016). Biological acidification of beer by non-conventional yeasts. Food Microbiology, 56, 1–10. https://doi.org/10.1016/j.fm.2015.11.010
Domizio, P., Romani, C., Lencioni, L., Comitini, F., Gobbi, M., Mannazzu, I., & Ciani, M. (2017). Outlining a method to produce beer with a controlled acidic profile using Lachancea thermotolerans. International Journal of Food Microbiology, 248, 20–29. https://doi.org/10.1016/j.ijfoodmicro.2016.12.022
Hranilovic, A., Gambetta, J. M., Schmidtke, L. M., Boss, P. K., Grbin, P. R., & Masneuf-Pomarede, I. (2018). Oenological traits of Lachancea thermotolerans show marked strain variation and convergence with Saccharomyces cerevisiae. FEMS Yeast Research, 18(3), foy008. https://doi.org/10.1093/femsyr/foy008
Varela, C., & Borneman, A. R. (2017). Yeast strain development for lower alcohol and sour beer production. Current Opinion in Biotechnology, 49, 121–129. https://doi.org/10.1016/j.copbio.2017.08.003
Ana Victoria Vasquez de la Peña
ana@neumaker.com.au
26th September 2025
© 2025 neumaker. All rights reserved. This article may be shared/forwarded for personal or educational purposes, provided it remains unaltered and includes proper attribution. Reproduction, distribution, or use in any other form—including but not limited to commercial purposes, republishing, or adaptation—without explicit written permission is strictly prohibited.